Hydroxyethyl Cellulose of daily-chemical grade has good mildew-resistant performance, system thickening and rheology modifying functions, as well as good water retention and film formation, and gives the final product full visual effects and all necessary application performance. The surface-treated Hydroxyethyl Cellulose has cold water solubility, and dry powder can be used and directly added into water. Good dispersion of the product in water can avoid clumping of product, and the occurrence of uneven dissolution. The final aqueous solution is uniform, continuous and full.
The Tg of HPMC not only influences its physical and chemical stability but also impacts its application in formulations. For instance, in pharmaceutical applications, the Tg is a determining factor for the stability of drug formulations, as it can affect the release rate of active pharmaceutical ingredients (APIs). If the Tg is too low, the material may become overly soft at room temperature, leading to difficulties in maintaining the integrity of solid dosage forms. Conversely, a Tg that is too high can impede the dissolution and bioavailability of drugs.
Manufacturers of redispersible latex powder often emphasize the versatility of their products, which are compatible with various types of binders, including cement, gypsum, and other mineral-based materials. This adaptability makes these powders suitable for a wide range of applications, from residential buildings to large-scale infrastructure projects.
As the pharmaceutical industry continues to evolve, the role of HPMC manufacturers is becoming increasingly vital. Their commitment to quality, innovation, and sustainability not only facilitates the development of effective drug delivery systems but also supports the industry's broader goals of improving patient outcomes. In a landscape driven by technological advancements and regulatory demands, HPMC manufacturers stand at the forefront, ensuring that pharmaceutical companies have access to the highest quality materials necessary for creating safe and effective medications. The future of drug development looks promising, with HPMC poised to play an essential role in shaping new therapies and improving healthcare worldwide.
The landscape of redispersible polymer powder manufacturers is dynamic and evolving, driven by innovation, quality control, customization, sustainability, and global outreach. As industries continue to seek high-performance materials, the role of these manufacturers will remain vital in providing solutions that enhance product capabilities. By understanding the intricacies of RDPs and the commitment of manufacturers to quality and sustainability, companies within the associated industries can make informed decisions, ultimately leading to improved product offerings and satisfied customers. Sustainable practices and innovation will undoubtedly shape the future of RDPs, paving the way for new possibilities in construction and beyond.
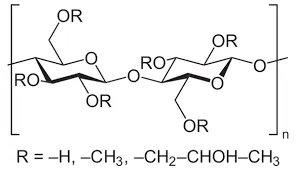
The primary raw material for HPMC synthesis is cellulose, a natural polymer derived from plant cell walls. Cellulose is abundant and renewable, making it an environmentally friendly choice. To initiate the synthesis, cellulose is first treated with an alkalizing agent, typically sodium hydroxide (NaOH), to create alkali cellulose. This step is crucial as it enhances the reactivity of cellulose by breaking down its crystalline structure.
Moreover, in the food industry, HPMC is often used as a thickening agent, stabilizer, or emulsifier. In these applications, its viscosity significantly influences the texture and mouthfeel of food products. A thicker consistency can enhance mouthfeel in sauces and dressings, while in baked goods, it can improve moisture retention and shelf life.